Earth is continuously bombarded by high energy protons, neutrons and electrons. The protons are quickly captured by the electrons to form hydrogen gas. Neutrons, however, travel deep into the atmosphere and collide with the nuclei of atoms in the lower atmosphere.
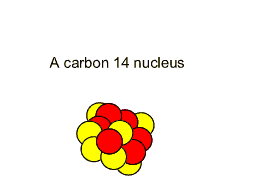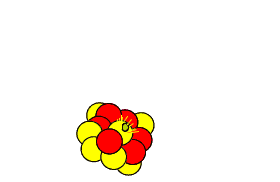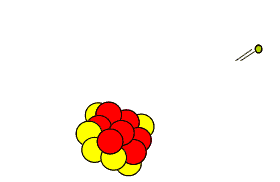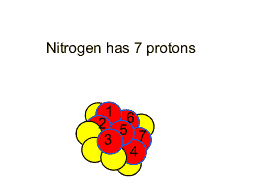
If a neutron is captured by the nucleus of a nitrogen atom a carbon atom is formed with atomic mass of 14 and a proton is ejected. This is shown by the word equation below.
Nitrogen-14 + neutron => carbon-14 + proton
Before we continue
it is important to be familiar with:
-atomic structure
-atomic number
-atomic mass
-isotopes
Carbon-14 is extremely unstable and decays back to nitrogen-14 releasing an electron in the process. Carbon-14 decays at a steady rate and the process is shown on the right.
Most of the carbon that exists on earth is in the form of carbon-12. Carbon-12 is extremely stable unlike its cousin carbon-14.
Because of the cosmic bombardment less than one-millionth of 1% of all the carbon on earth is in the form of carbon-14. Since all living organisms are carbon based creatures, a little carbon-14 is present in all plant and animal life on earth. Living organisms have a fixed ratio of carbon-12 to carbon-14 present in their bodies. Once the plant or animal dies the carbon-14 decays to nitrogen-14 at a steady rate. The longer an organism is dead the lower the carbon-14 content.
Every 5,730 years half of the carbon-14 present will decay to nitrogen-14. This is known as the half-life of carbon-14.
Let's take an example.
An archeologist extracts a gram of carbon from an ancient bone and measures
the emitted radioactivity at between 15 to 16 beta emissions per minute.
A gram of carbon was also extracted from the bones of an animal that had
recently died and the emission recorded at 62 beta emissions per minute.
Estimate the age of the bones.
Beta emission for the old sample is one quarter that of the fresh sample. Two half-lives have passed thus the amount of time elapsed is 11,460 years.
Wood from an ancient boat was sampled. A one gram carbon sample was obtained and beta emissions recorded. It was found that the emissions were 12.5% of those from a one gram sample of a fresh piece of wood. How old is the boat estimated to be?
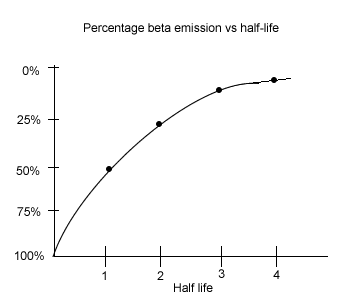
From the graph on the right we can see that 3 half-lives must pass for the emissions to reduce to 12.5% of the original. The boat is estimated to be 17,190 years old.
1) What is meant by half-life?
2) What happens to the atomic number of carbon-12 when it ejects a beta
particle? Does its atomic mass change?
3) Why would carbon dating methods be useless in dating old silver coins
but not in dating wood carvings from the same era?
4) How are isotopes of carbon-12 and carbon-14 different? How are they
similar?
5) A bone fragment from an ancient burial site was unearthed and analysed.
It was found to contain isotopes of carbon-14 and nitrogen-14 in the
ratio 9:1 respectively. How old is the bone? Given a 15% uncertainty
what is the oldest time period for this animal to have walked on earth?
6) The useful range for carbon dating is between 100 and 30,000 years.
Explain why?
7) Potassium-40 has a half-life of 1.3 billion years and decays to argon-40.
a) How long will it take for 20 grams of potassium-40 to decay to 5
grams?
b) What is given off by potassium-40 to decay to argon-40?
c) How does the atomic mass and atomic number of potassium-40 change
as it decays to argon-40?